

Negative ions in the body relax you and energize your body. Ions are sources of energy, whether in the human body or electrical gadgets. Some examples of molecular ions are NH 4 + (ammonium), OH – (hydroxide), etc. Similar to an atomic ion, a molecular ion may be molecular cation or anion.
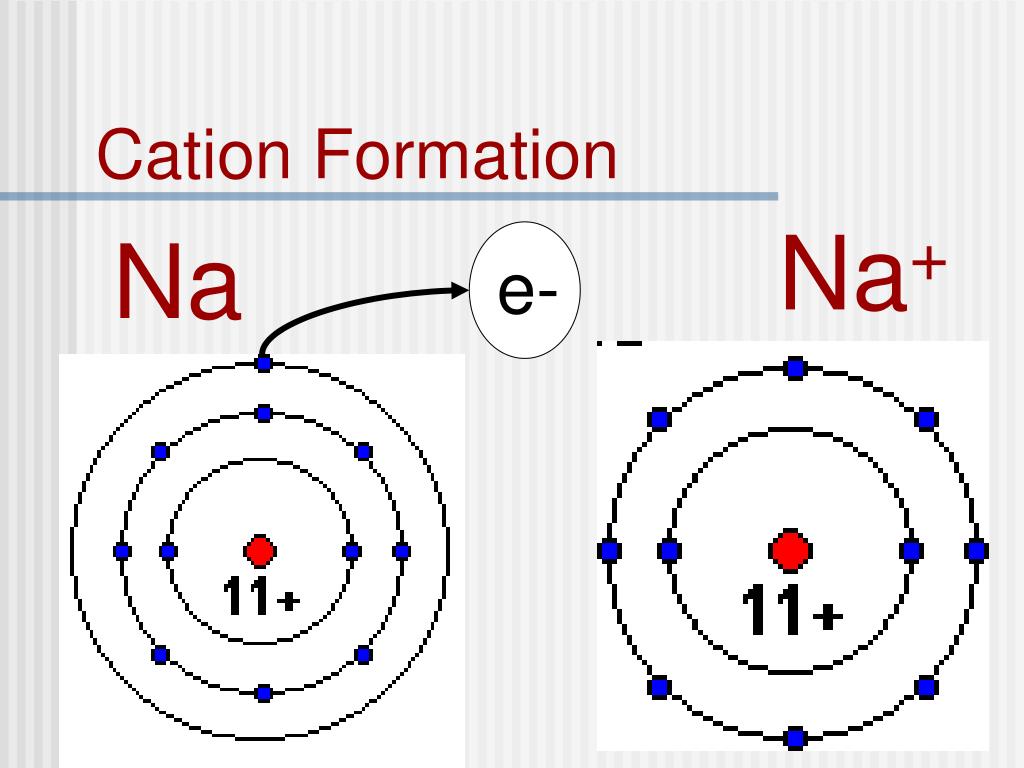
When a molecule (a group of atoms bonded together) combines with an atomic ion, the molecule gets charged and becomes an ion. So atoms find it easier to lose or gain electrons to become ions. An ion or group of ions having a negative charge and characteristically moving toward the positive electrode in electrolysis is called an anion. Protons are 1836 times heavier than electrons. Atom can gain of proton to get positively charged.Atom can lose a proton to get negatively charged.Atom can gain an electron to get negatively charged.Atom can lose an electron to get positively charged.There are 4 ways in which an atom can become an ion: This negatively charged ion is called anion. When an atom has more electrons than protons, it gets negatively charged. This positively charged ion is called cation. When an atom has more protons than neutrons, it gets positively charged. When the number of these charged particles is the same, the atom is neutral. So atoms are made up of charged particles (and also neutral ones). The protons and neutrons form the nucleus of the atom whereas electrons orbit around the nucleus, as shown in Figure 1.įigure 1: Electrons Revolving Round the Nucleus they have neither negative nor positive charge. Electrons are negatively charged while protons are positively charged. Sub-atomic particlesĪtom is made up of electrons, protons and neutrons. Alternately, we can say that atom is the smallest unit of matter that can exist in a stable form. As we discussed in a previous post, all matter is made up of atoms. A negatively charged ion, which has more electrons in its electron shells than it has protons in its nuclei, is known as an anion, for it is attracted to. All rights reserved.Ions are nothing but atoms or molecules with positive or negative electrical charge. Copyright © 2023, Columbia University Press. View solution > Select the correct answer from the given choices for the statement given below. View solution > An ion with positive charge is called. View solution > Fill in the blanks: Negatively charged ions are called. Commonly, anions and cations are called ions. A negatively charged ion in a compound is called : Easy. The Columbia Electronic Encyclopedia, 6th ed. The key difference between anion and cation is that anions are the negatively charged ions formed from neutral atoms whereas cations are positively charged ions formed from neutral atoms. In the salt sodium carbonate, Na 2CO 3, two sodium cations are needed to neutralize each carbonate anion, CO 3 −2, because its charge is twice that of the sodium ion. (Image from here.) Its a fairly simple concept for an atom to become an ion. In common table salt, or sodium chloride, NaCl, the sodium cations, Na +, are neutralized by chlorine anions, Cl −. Salt, NaCl, contains positively and negatively charged atoms called ions. For example, when NaCl dissolves into Na + and Cl - in water, Cl - is an anion because it gained an electron. Since ordinary matter is electrically neutral, ions normally exist as groups of cations and anions such that the sum total of positive and negative charges is zero. Answer and Explanation: A negative ion is called an anion. How are chlorine ions formed The chloride ion /klrad/ is the anion (negatively charged ion) Cl. Salt, NaCl, contains positively and negatively charged atoms called ions. These atoms which are not neutral in charge are called ions. Ionic bonding is the attraction between positively- and negatively-charged ions. Since electrons are negatively charged, an atom that loses one or more electrons will become positively charged an atom that gains one or more electrons becomes negatively charged. If an atom or group gains electrons or loses protons, it will have a net negative charge and is called an anion. The compound sodium chloride has a positively charged sodium atom and a negatively charged chloride atom. Why do ions exist Ions form when atoms gain or lose electrons. If an atom or group loses electrons or gains protons, it will have a net positive charge and is called a cation. A simple ion consists of only one charged atom a complex ion consists of an aggregate of atoms with a net charge. Since the electron and proton have equal but opposite unit charges, the charge of an ion is always expressed as a whole number of unit charges and is either positive or negative. A neutral atom or group of atoms becomes an ion by gaining or losing one or more electrons or protons.


 0 kommentar(er)
0 kommentar(er)
